Discovery - Neurolipidomics: Mapping the neural lipidome
RESEARCH DISCIPLINES: BIOCHEMISTRY, NEUROSCIENCE, CHEMISTRY; RESEARCH AREAS: NEURODEGENERATIVE DISEASES (ALZHEIMER'S DISEASE, PARKINSON'S DISEASE, VASCULAR DEMENTIA, CEREBROVASCULAR ACCIDENT, RARE PEDIATRIC DISEASES), CHRONOBIOLOGY, LIPIDOMICS, BIOINFORMATICS
Why are subpopulations of neurons vulnerable in Alzheimer's Disease (AD) and Parkinson's Disease (PD)? Advances in genomics have enabled researchers to identify genetic determinants of early onset disease. Direct biochemical investigations have elucidated multiple signaling pathways altered by these mutant gene products. Further combination of genomics with proteomics is allowing researchers to map global gene and protein changes associated with progressive neurodegeneration. Together, these studies have provided remarkable insight into the molecular nature of AD and PD. Despite these advances, we do not know why discrete subsets of cells are uniquely susceptible to early dysfunction nor can we protect vulnerable populations. Here, we argue that the next major advance in rational therapeutic design will come from tying a cell's metabolome into genomic and proteomic maps of disease. We argue that a "targeted systems approach" will provide a new understanding into the metabolic regulatory mechanisms that determine why groups of cells become transiently susceptible and finally succumb to genetic and environmental determinants of AD and PD. Such insight will provide new therapeutic avenues designed to protect vulnerable neuronal populations in the face of ongoing insult.
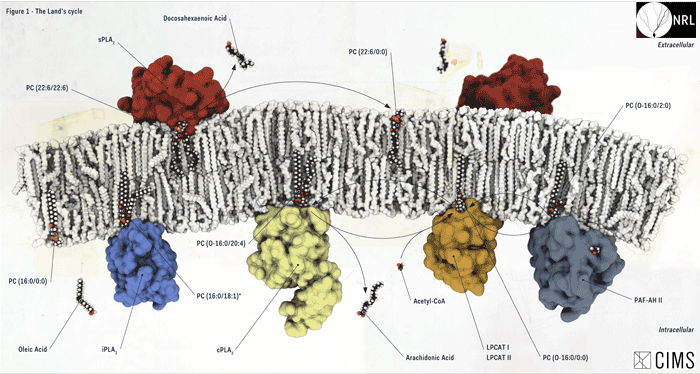
Why lipids? Lipids are the building blocks of biological membranes and make up half the wet weight of brain tissue. They modulate membrane trafficking. They transduce intracellular signalling pathways. While commonly studied in families (e.g., diacylglycerols, glycerophospholipids, cholesterols), individual isoforms display unique properties that differentially regulate cellular responses to external and internal stimuli. Certainly, AD and PD are, in large part, mediated by changes in lipid metabolism with disruptions in glycerophospholipid, cholesterol, arachidonic acid, and phosphatidylinositol-4,5-bisphosphate metabolism associated with accelerated cognitive decline. Mechanistic insight, however, has been complicated by the considerable technical challenges associated with distinguishing pathogenic from non-pathogenic lipid species within samples containing several thousand isoforms.
The field of lipidomics seeks to address this issue. Lipidomics defines "the large-scale analysis of lipid profiles in cells and tissues". Neurolipidomics focuses on cellular, regional, and systemic lipid homeostasis in the central nervous system encompassing not only the identification and measurement of individual lipid isoforms but also the mRNA and protein expression profiles of metabolic enzymes and transporters and the protein targets that affect downstream signalling in neurons and glia. Further, a lipidomic analysis also includes an unbiased assessment of lipid function ranging from the physicochemical basis of lipid behaviour to lipid-protein and lipid-lipid interactions, and impact of dynamic lipid metabolism on cellular response to intrinsic and extrinsic stimuli.